Abstract

Introduction Off-the-shelf allogeneic T-cell therapies face the major challenges of graft-versus-host disease (GVHD) and graft rejection mediated by host and recipient alloreactive T-cells respectively. To address GVHD, we are using Epstein-Barr Virus-specific T cells (EBVSTs), which are virus specific rather than allo-specific and have not produced GVHD in over 300 allogeneic recipients. To prevent graft rejection, we modified the EBVSTs with a chimeric antigen receptor targeting CD30, an antigen that is upregulated on allo-activated T-cells, which will consequently become targets for CD30.CAR EBVSTs. Hence, CD30.CAR EBVSTs can kill both CD30+ lymphoma cells and alloreactive T-cells through their CAR, and may be boosted in vivo in EBV+ recipients via their native TCR. We are evaluating this approach in a Phase I/II trial (NCT04288726) in patients with CD30-positive lymphoma.
Methods We generated a bank of seven CD30.CAR EBVST lines by stimulating peripheral blood mononuclear cells (PBMC) from healthy donors with overlapping EBV peptide mixtures (pepmixes) followed by retroviral transduction with a second generation CD30.CAR containing a CD28 costimulatory endodomain. We have treated 14 patients (median age 36, range 22-53) with relapsed or refractory Hodgkin's lymphoma. Patients had undergone a median of 5 (range 3-6) prior lines of treatment. Escalating doses of 4 × 107 (DL1), 1 × 108 (DL2) or 4 × 108 (DL3) CD30.CAR EBVSTs were infused after lymphodepletion with cyclophosphamide and fludarabine. Blood drawn pre- and post- infusion was processed to isolate serum/plasma and PBMC for analysis. The frequency of CD30.CAR EBVSTs was measured by real time qPCR for the transgene. Changes in the frequency of T-cells responding to EBV or tumor-associated antigens (epitope spreading) were measured by IFNɣ ELISpots. Clinical responses were assessed by diagnostic PET/CT scans performed 4-8 weeks post infusion.
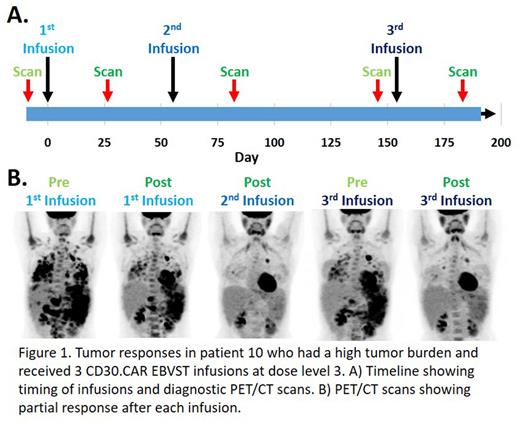
Results CD30.CAR EBVSTs were well tolerated, with no GVHD and only two patients having reversible grade 4 cytopenia. Four patients at DL3 had grade 1 cytokine release syndrome (CRS), but all resolved without treatment. Of the 14 patients treated, 13 have been evaluated for responses per Lugano criteria; 4 had partial responses (PR) and 5/10 patients at DL2 and DL3 had complete responses (CR) giving an overall objective response rate (ORR) of 69.2%. Despite these remarkable responses, qPCR for the CD30.CAR transgene showed near background levels by week 1 post infusion. Possible explanations are that CD30.CAR EBVSTs; (1) Are eliminated by alloreactive T-cells, but persist sufficiently to eliminate tumors. (2) Can reactivate endogenous tumor-specific T-cells that are responsible for the tumor responses. (3) Rapidly eliminate tumors and are then lost due to being intrinsically short-lived. (4) Become tissue resident at the tumor site. We think explanation #1 cannot be a major contributor to the lack of circulating CD30CAR T cells since patient 10, who had bulky disease received 3 infusions from the same donor line and responded after each infusion as shown in Figure 1; an alloreactive response would be anticipated to be amplified after each infusion. Similarly, two additional patients receiving second infusions both achieved CR. While we cannot exclude explanation #2, we did not detect epitope spreading in patient PBMCs that would be expected if responses after infusion of CD30.CAR EBVSTs were primarily attributable to reactivation of host anti-tumor cells. We are therefore pursuing explanations #3 and #4 by analyzing tumor biopsy samples to determine the presence of CD30.CAR EBVSTs at tumor sites and characterize their phenotype for functionality.
Conclusion We have shown CD30.CAR EBVSTs can be a safe and effective treatment for CD30+ lymphomas, and may avert GVHD and immediate rejection even after multiple infusions. We now seek to improve the durability of responses and test whether CD30.CAR EBVST can be used as a platform for other "off-the-shelf" CAR-T cell therapies.
Disclosures
Quach:Tessa Therapeutics: Research Funding. Ramos:Tessa Therapeutics: Consultancy, Patents & Royalties: HPV-specific T cell manufacture, Research Funding; Athenex Therapeutics: Consultancy, Research Funding; Genentech: Consultancy; Novartis: Consultancy; CRISPR Therapeutics: Consultancy. Grilley:Allovir: Consultancy, Current equity holder in publicly-traded company, Other: Leadership, Regulatory guidance and support; QB Regulatory Consulting: Other: Ownership, Project management support, Research Funding; Tessa Therapeutics: Consultancy, Other: Regulatory guidance and support; March Bio: Consultancy. Brenner:Abintus Bio: Membership on an entity's Board of Directors or advisory committees; Bellicum Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; TScan Therapeutics: Membership on an entity's Board of Directors or advisory committees; Memgen: Membership on an entity's Board of Directors or advisory committees; KUUR: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees, Other: Stock options; Walking Fish Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Stock options; Poseida Therapeutics: Membership on an entity's Board of Directors or advisory committees; Adaptimmune Therapeutics: Membership on an entity's Board of Directors or advisory committees; Coya Therapeutics: Membership on an entity's Board of Directors or advisory committees; Marker Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Tessa Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Turnstone Biologics: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees. Heslop:GSK: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Ankarys: Membership on an entity's Board of Directors or advisory committees; Immunai: Membership on an entity's Board of Directors or advisory committees; Gilead Biosciences: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Kuur Therapeutics: Research Funding; Allovir: Current equity holder in publicly-traded company; Marker Therapeutics: Current equity holder in publicly-traded company; Kiadis: Divested equity in a private or publicly-traded company in the past 24 months; Fresh Wind Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Rouce:Tessa Therapeutics: Research Funding; Novartis: Honoraria; Kite Pharmaceuticals: Research Funding; Pfizer: Consultancy. Lapteva:Tessa Therapeutics: Consultancy. Rooney:Bellicum Pharmaceuticals: Patents & Royalties; Tessa Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Santa Ana Bio: Research Funding; Marker Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties; Allovir: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal